At UTHealth, a Pioneering Prostate Cancer Treatment
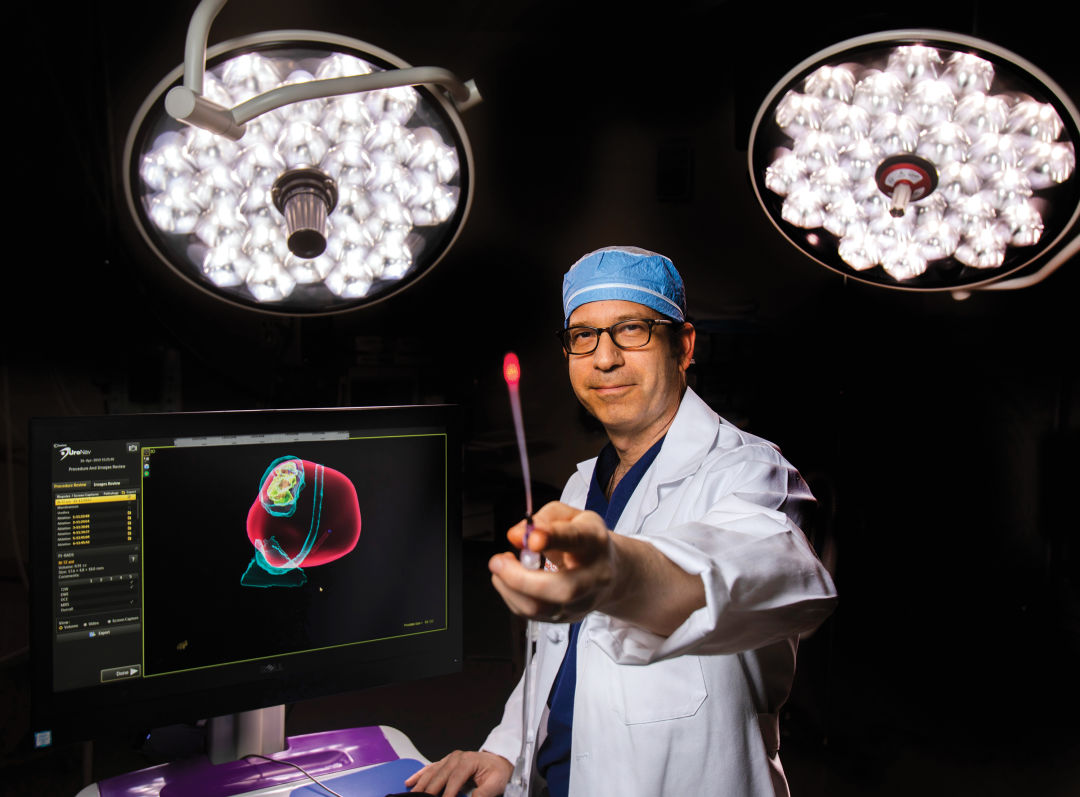
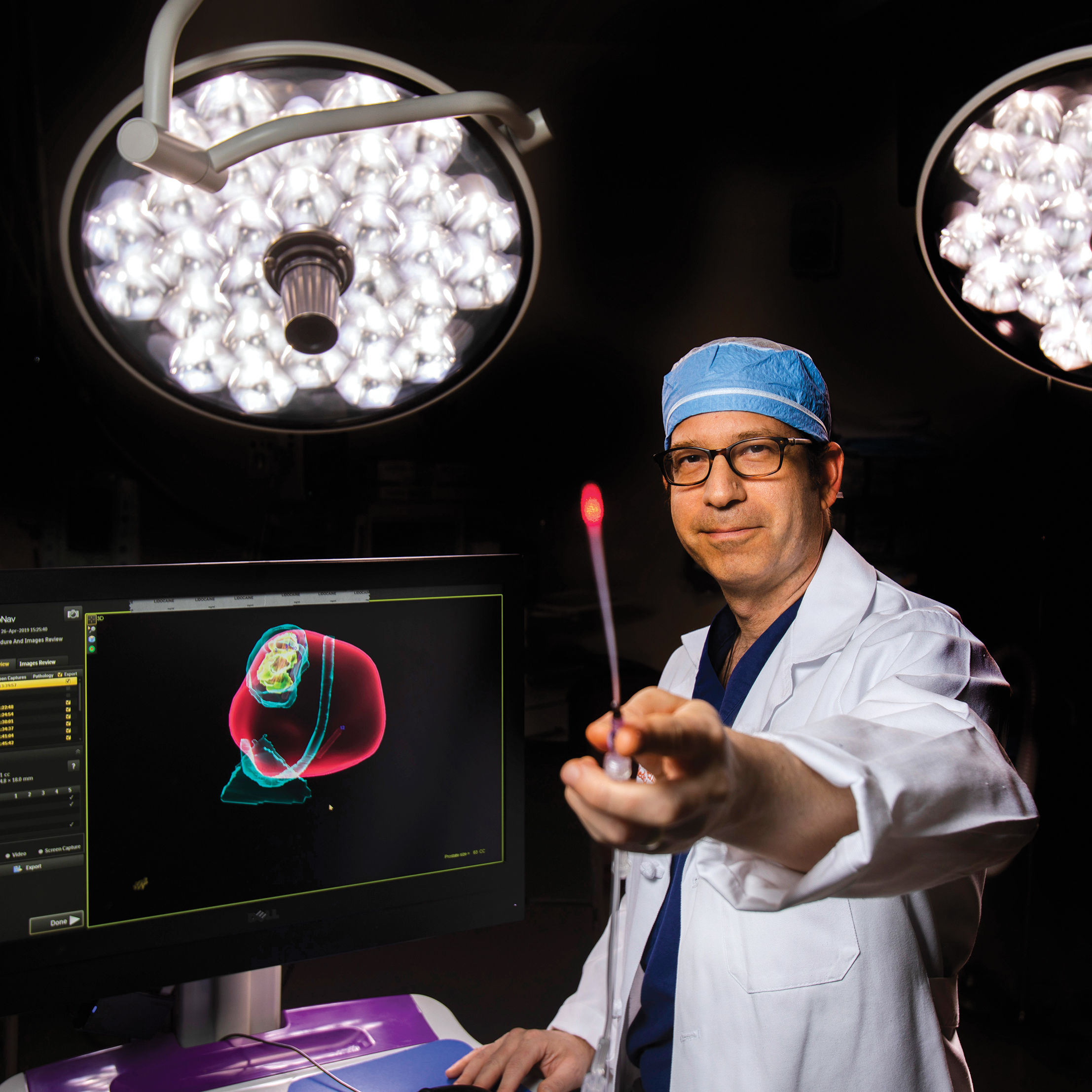
Dr. Steven Canfield is conducting clinical trials on a procedure that could revolutionize prostate cancer treatment in the next decade.
Image: Daniel Kramer
Charles Flewellen grew up in a medical family. So when his doctor found an enlargement on his prostate in 2017, the League City resident knew he would be facing some tough decisions.
“There was a time when you died from it,” says Flewellen, who is now 65 and retired from a career in IT. Today, though, the odds are much better: The death rate from prostate cancer has been cut in half since the early 1990s, according to the American Cancer Society, while the five-year survival rate among men with localized prostate cancer is nearly 100 percent. Nevertheless, it remains the most common cancer in American men, affecting 1 in 9, and treatment comes with a price.
Flewellen’s family doctor referred him to Dr. Steven Canfield, chief of urology at Memorial Hermann–Texas Medical Center and chief of the urology division at UTHealth Science Center’s McGovern Medical School. Canfield presented Flewellen with two standard treatment options: to monitor the situation and do periodic biopsies, or opt for a radical prostatectomy. The former meant regular and inconvenient procedures, while the latter—a surgery that removes the prostate completely—could lead to problems with urination and sexual function.
When Flewellen told the doctor that he didn’t like either choice, Canfield offered a third option. He was beginning clinical trials for an innovative method to treat the disease. If Flewellen agreed to participate, his blood would be infused, via IV, with microscopic gold nanoparticles—small spheres of silica wrapped in thin layers of gold that travel through the body, entering the cells of highly inflamed areas such as tumors. The surgeon, viewing the prostate via MRI, would then use a laser to activate the nanoparticles, which vibrate when subjected to near-infrared light. The hope? That they would kill the affected cells without damaging the healthy tissue around the tumor, and without causing lasting side effects. Flewellen signed on.
Canfield, who’s been with UTHealth for 14 years, started working on medical applications of gold nanoparticles a decade ago. While infusing the bloodstream with gold is known to be safe, his team—including Naomi Halas, head of Rice University’s nanophotonics lab, and inventor of gold nanoparticles—had to wait for prostate cancer imaging technology to improve to try the procedure.
“If you’re trying to apply a focal therapy, it only works if you can localize the cancer, so that was the problem,” says Canfield. “We knew that the nanoparticles could find the cancer, since that was something proven in earlier studies … But you can’t see nanoparticles with your eyes or with an X-ray, because they’re too small.”
By 2016 the technology had arrived. Canfield and his team met with physicians and researchers at other cancer centers across the country to develop a study, and in May 2017 began clinical trials. Flewellen was the first patient in Texas to undergo treatment.
The infusion, which Canfield compares to receiving saline through an IV, lasted about two hours, after which Flewellen was able to go home. The next day, once the nanoparticles had settled, he underwent a three-hour laser treatment and again went home. Two days later an MRI indicated that the treatment was already working, and after three months, a follow-up scan showed that the cancer had been eliminated. Flewellen's MRI is still clear six months after the procedure.
“It puts tingles up and down my back to think about it,” he says. “It gave me a different choice. If this problem came up a few years ago, there would’ve been nothing, no other options for us. We would have had to take the extreme option or chemo, or heaven knows what.”
Canfield, of course, will continue to keep an eye on his patient. “We haven’t removed the whole prostate,” says the doctor. “There could be cancer that develops in other parts of the prostate someday, so they have to be monitored, but we reset the clock.”
The first round of trials for the treatment continues at UTHealth, the Icahn School of Medicine at Mount Sinai, and the University of Michigan, with a second round launching soon. Once that’s finished, Canfield hopes the FDA will approve the procedure, which he feels confident will be a game changer.
“Focal therapy, I think, will be the paradigm shift moving into the future decade for prostate cancer, and there has to be a really good way to deliver focal therapy in a very precise way, better than what has been developed so far,” says Canfield. “This looks like it will fulfill that promise.”