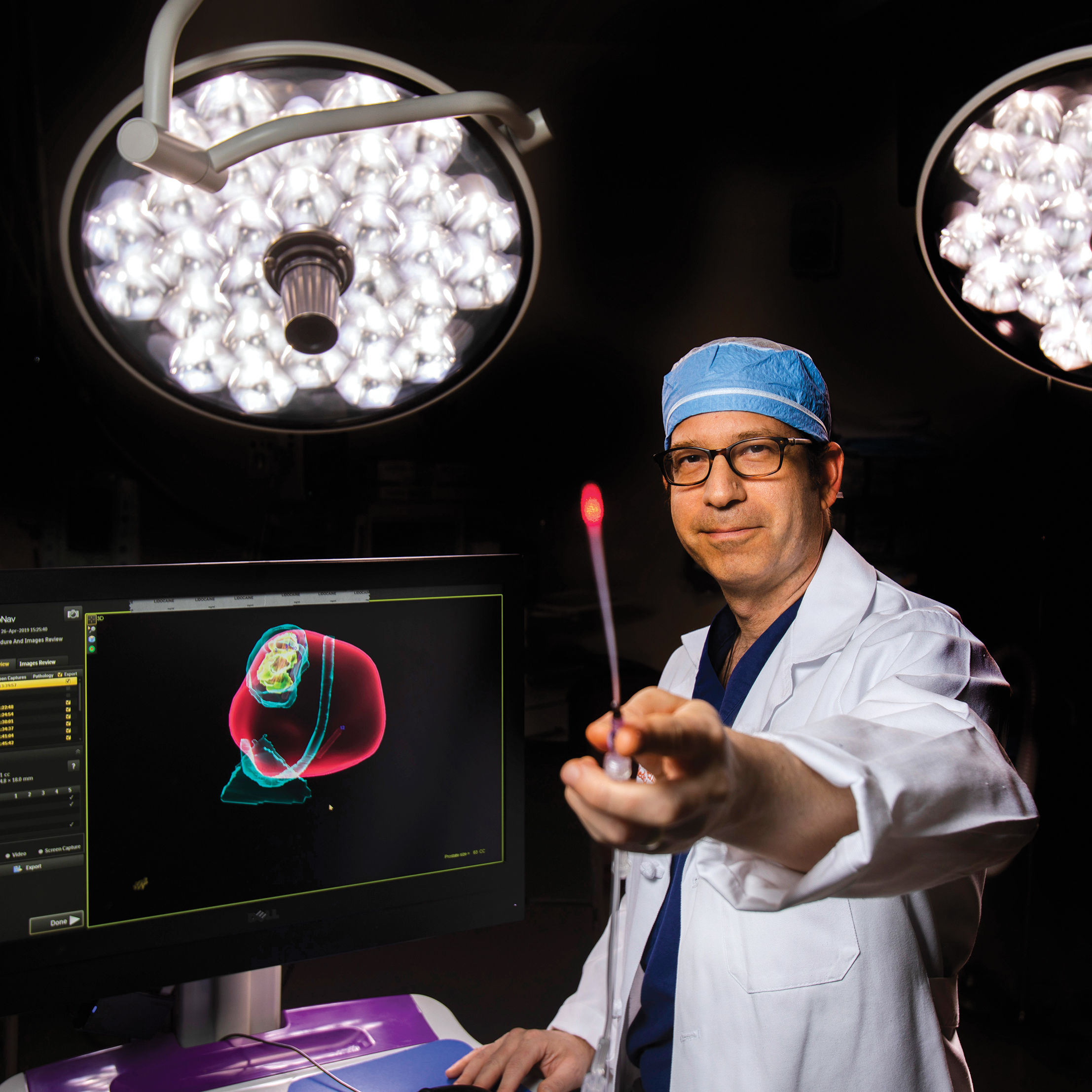
At Houston Methodist, an Attempt to Reverse Aging on a Cellular Level

Dr. John Cooke is pioneering a treatment that could slow, and possibly reverse, progeria.
Image: Daniel Kramer
Scientist Genevieve Bertolet pulls up a picture of two glowing, green nuclei on her laptop, images that may hold the key to reversing the aging process. The healthy one appears as an oval. The other, belonging to the cell of a child with Hutchinson-Gilford Progeria Syndrome, looks flopped over on itself, deflated.
Progeria is a genetic disease that affects 1 in 20 million people, or about 400 patients worldwide. Caused by a protein mutation in the connective tissue that holds cell nuclei together, it rapidly accelerates aging in children: Those who suffer from it don’t live much beyond 14 years old, when their hearts give out. But inside the Center for Cardiovascular Regeneration’s laboratory at the gleaming Houston Methodist Research Institute in the Texas Medical Center, the team of cutting-edge researchers at the hospital’s RNAcore initiative is attempting to change that.
“It’s because of this connective tissue, this cement,” says Bertolet’s boss, Dr. John Cooke, who chairs Methodist’s cardiovascular science department and runs RNAcore. “When it’s mutated, it causes changes to the genomic architecture of the chromosomes. You can see these changes. They resemble changes that occur with aging cells.”
Progeria currently has no cure, and until recently patients had only one treatment option, a drug originally developed to fight cancer that can prolong their lives by a year or two. But Cooke has an idea for a revolutionary approach that would do much more than that, using RNA therapeutics—treatment that is focused on ribonucleic acids, a substance found in all living cells—to slow, and possibly reverse, the disease.
In 2015 Cooke applied for a grant from the Progeria Research Foundation, a nonprofit that has been researching the disease since Dr. Leslie Gordon established it in 1999, the year after her son Sam was diagnosed. By 2003 the foundation had discovered its cause—an abnormal protein, progerin—and since then has been funding research in hopes of finding a cure. “A primary requirement for all of our grants,” says Gordon, “is we have to see a pathway within a reasonable number of years from something tangible to a cause, treatment, and cure for progeria. Dr. Cooke beat out a lot of grant applications.”
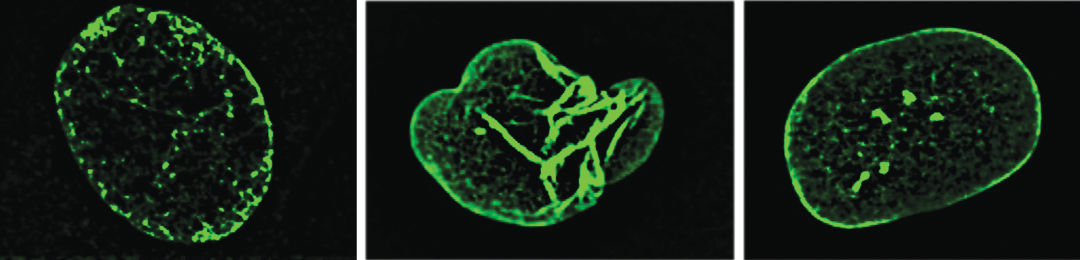
Progeria, caused by a genetic mutation in cells' nuclear laminate, accelerates aging children. Pictured: a normal nucleus, left; one with the mutation, middle; and one that's been treated, right.
Image: Courtesy of John Cooke
Cooke and his team have been studying the disease since 2017. Their research focuses on the ends of chromosomes, or telomeres, which he refers to as “the timekeepers of cells.” These act like the tips of shoelaces, keeping chromosomes from fraying apart and allowing them to function properly. As we age telomeres erode, shortening every time a cell divides and deteriorating when the body is under stress. In children with progeria, Cooke has discovered, this process is vastly accelerated. By the time patients reach their early teens, their telomeres resemble those of a 65-year-old adult.
Would lengthening them slow aging on a cellular level? Cooke’s team used cells provided by the PRF, belonging to progeria patients ranging in age from 1 to 16 years, to find out. They developed a ribonucleic acid that acts as a therapeutic agent, directing progeria cells to make a protein—telomerase—that extends the length of the telomere in a series of preclinical studies.
The research has proved to be groundbreaking. The team has found that the process makes a difference in all cell function, including proliferation and division, and that there’s a direct link between the progerin protein and telomere length. “It’s biological software,” says Cooke. “We have cells of the children. We’ve found we can restore the health of those cells. We can literally reverse aging in those cells.”
Because the aging process in progeria patients is similar to what occurs in all of us—just accelerated—the new therapy could have other implications. “It could be used for a lot of age-based disorders,” Cooke says, even preventing heart attacks or strokes, which might lead to longer, healthier lives for a wide range of people.
For now, though, Cooke is concentrating on progeria patients. Preclinical studies, in which the therapy will be tested on mice with the mutation, are now starting, and he hopes to get the go-ahead to start clinical trials soon—maybe by next year. “What we’re focused on is improving people’s health,” he says. “We’re moving toward a therapy that will be transformational for these children. I do believe that.”